Product History
From our origins as a domestic vaccine manufacturer, we continue to push medical boundaries to support the health needs of people around the world.
Last updated: March, 2019

Beginning vaccine production
The poor public sanitation and hygiene that followed World War II propelled us to begin producing vaccines for smallpox and cholera, a combined vaccine against typhus and paratyphoid, and a tetanus antitoxin.

Beginning vaccine production
The poor public sanitation and hygiene that followed World War II propelled us to begin producing vaccines for smallpox and cholera, a combined vaccine against typhus and paratyphoid, and a tetanus antitoxin.

Approval of our DPT vaccine
Our DP vaccine was approved in 1959, followed by our DPT vaccine in 1964. We continued to research ways to improve this vaccine, culminating in the development and approval of a DTaP vaccine in 1981, using a groundbreaking pertussis component vaccine with significantly reduced impurities. This was followed by the development and approval of DTaP‐IPV in 2012. And in 2019 we continue to improve this vaccine by conducting clinical trials for a DTaP‐IPV‐Hib pentavalent vaccine.

Approval of our DPT vaccine
Our DP vaccine was approved in 1959, followed by our DPT vaccine in 1964. We continued to research ways to improve this vaccine, culminating in the development and approval of a DTaP vaccine in 1981, using a groundbreaking pertussis component vaccine with significantly reduced impurities. This was followed by the development and approval of DTaP‐IPV in 2012. And in 2019 we continue to improve this vaccine by conducting clinical trials for a DTaP‐IPV‐Hib pentavalent vaccine.

Launch of Bimmugen – the first Japanese recombinant Hepatitis B vaccine (yeast derived)
The technologies and strategies gained during the development of Bimmugen have become the basis of our recombinant product development strategy. The successful development of Bimmugen led to enduring trust in our products and provided opportunities for pharmaceutical research and development.

Launch of Bimmugen – the first Japanese recombinant Hepatitis B vaccine (yeast derived)
The technologies and strategies gained during the development of Bimmugen have become the basis of our recombinant product development strategy. The successful development of Bimmugen led to enduring trust in our products and provided opportunities for pharmaceutical research and development.
Launch of BOLHEAL – the first Japanese fibrin sealant
BOLHEAL is indicated for use in tissue adhesion and closure. Clinical trials showed that BOLHEAL is effective in surgeries when it is difficult to use sutures or join tissue together, and in surgeries that carry the risk of bleeding or leakage of bodily fluids and gases.
Launch of BOLHEAL – the first Japanese fibrin sealant
BOLHEAL is indicated for use in tissue adhesion and closure. Clinical trials showed that BOLHEAL is effective in surgeries when it is difficult to use sutures or join tissue together, and in surgeries that carry the risk of bleeding or leakage of bodily fluids and gases.

Launch of AnactC – the world’s first activated protein C product derived from human plasma
AnactC is indicated for the treatment of conditions caused by congenital protein C deficiency, such as deep vein thrombosis, acute pulmonary thromboembolism, and purpura fluminans.

Launch of AnactC – the world’s first activated protein C product derived from human plasma
AnactC is indicated for the treatment of conditions caused by congenital protein C deficiency, such as deep vein thrombosis, acute pulmonary thromboembolism, and purpura fluminans.

Succeeded in full‐length cDNA cloning of ADAMTS13
We successfully cloned the cDNA coding of ADAMTS13, an enzyme that cleaves von Willebrand factor (VWF), which has been identified as the causative protein of congenital thrombotic thrombocytopenic purpura (TTP). We have licensed the gene patent to a partner and are collaborating in international clinical trials for a recombinant ADAMTS13 product for the treatment of TTP.

Succeeded in full‐length cDNA cloning of ADAMTS13
We successfully cloned the cDNA coding of ADAMTS13, an enzyme that cleaves von Willebrand factor (VWF), which has been identified as the causative protein of congenital thrombotic thrombocytopenic purpura (TTP). We have licensed the gene patent to a partner and are collaborating in international clinical trials for a recombinant ADAMTS13 product for the treatment of TTP.

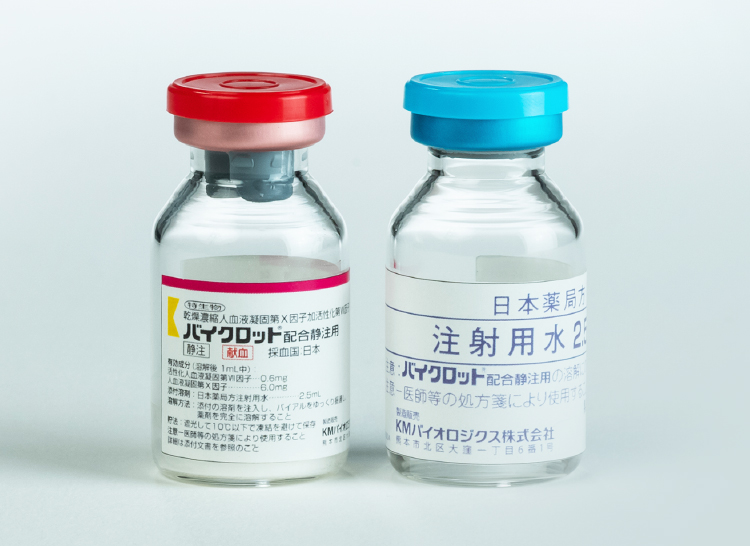
Launch of a new haemostatic treatment for haemophillia patients with inhibitors: Byclot – human coagulation factor VIIa containing factor X
Byclot is indicated for the control of bleeding episodes in patients with blood coagulation factor VIII or factor IX deficiency with inhibitors.

Launch of a new haemostatic treatment for haemophillia patients with inhibitors: Byclot – human coagulation factor VIIa containing factor X
Byclot is indicated for the control of bleeding episodes in patients with blood coagulation factor VIII or factor IX deficiency with inhibitors.

Approval of our cell culture Influenza vaccine
We successfully developed a pandemic influenza vaccine using a cell culture method to complement our traditional egg‐based influenza vaccine.

Approval of our cell culture Influenza vaccine
We successfully developed a pandemic influenza vaccine using a cell culture method to complement our traditional egg‐based influenza vaccine.