Blood Plasma Products
Our products are made from blood plasma donated in Japan. As part of our work process, we perform virus testing on plasma, as well as the inactivation and removal of viruses during the manufacturing process. We also perform virus tests on final products to ensure the safety of all our blood plasma products. Under Japanese regulations, plasma-derived products cannot be exported.
History
The Japanese blood donation system began in 1964. The following year our intramuscular immunoglobulin was approved for use, followed by the approval of our albumin in 1966.
- 1979: Launched Confact8 (Coagulation Factor VIII/von Willebrand Factor Complex)
- 1980: Launched Venilon-I (Sulfonated Intravenous Immunoglobulin)
- 1985: Launched ConfactF (Coagulation Factor VIII/von Willebrand Factor Complex)
- 1991: Launched BOLHEAL (Fibrin Sealant)
- 1992: Launched NovactM (Coagulation Factor IX Preparation)
- 2001: Launched AnactC (Activated Protein C)
- 2014: Launched Byclot (Coagulation Factor VIIa Containing Factor X)
Anact C Injection 2500 units
(Freeze-dried Human Activated Protein C Concentrate)
Indications :
The following diseases caused by congenital protein C deficiency
- Deep vein thrombosis, acute pulmonary thromboembolism
- Purpura fulminans
Storage :
Do not freeze. Store at 10°C or below.
Shelf life :
3 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Immunoaffinity chromatography
- Viral filtration
- Dry heat treatment (65°C, 96h)
- Final product PCR (NAT) testing for HIV, HBV, HCV, HAV,and parvovirus B19.
BOLHEAL Tissue Sealant
(Human fibrinogen, Human antithemophilic factor XIII fraction, Apritinin Solution, Thronbin(JAN), Calcium Chloride Hydrate(JAN))
Indications :
Tissue adhesion and closure
Storage :
Do not freeze. Store at 10°C or below.
Shelf life :
30 manths
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Viral filtration
- Dry heat treatment (Thrombin: 65°C/96h, Fibrinogen: 65°C/144h)
- Final product PCR (NAT) testing for HIV, HBV, HCV, HAV,and parvovirus B19.
Byclot Combination I.V. Injection
(Freeze-dried Activated Human blood coagulatiion factor VII Concentrate containing factor X)
Indications :
Prevent or reduce the frequency of bleeding episodes in patients with blood coagulation factor VIII or factor IX inhibitors.
Storage :
Do not freeze. Store at 10°C or below.
Shelf life :
3 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- S/D processing
- Viral filtration
- Dry heat treatment (65°C, 96h)
- Final product PCR (NAT) testing for HIV, HBV, HCV, HAV,and parvovirus B19.
Confact F I.V. Injection 250 units, 500 units, 1000 units
(Freeze-dried Human blood coagulatiion factor VIII Concentrate)
Indications :
- Reduce the frequency of bleeding episodes in patients with blood coagulation factor VIII deficiency.
- Reduce the frequency of bleeding episodes in patients with von Willebrand disease.
Storage :
Do not freeze. Store at 10°C or below.
Shelf life :
3 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Viral filtration
- Dry heat treatment (65°C, 96h)
- Final product PCR (NAT) testing for HIV, HBV, HCV, HAV,and parvovirus B19.
Histaglobin S.C. Injection
Indications :
- Bronchial asthma
- Allergic rhinitis
- Vasomotor rhinitis
- Allergic skin diseases (urticaria, chronic eczema, and atopic dermatitis)
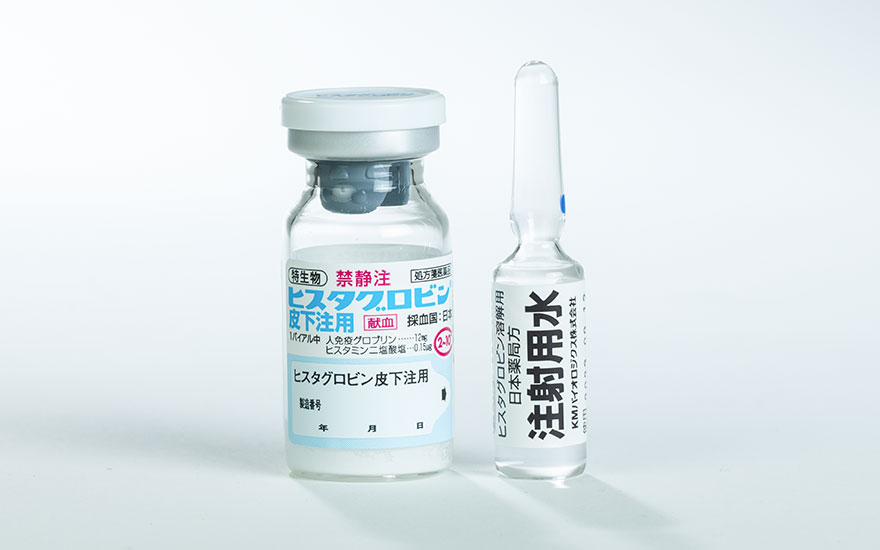
Storage :
Do not freeze. Store between 2°C and 10°C
Shelf life :
3 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Ethanol fractionation
- Viral filtration
Kenketsu Albumin 20% I.V. Injection 10g/50mL “KMB”
Kenketsu Albumin 25% I.V. Injection 12.5g/50mL, 25g/100mL “KMB”
(Human Serum Albumin)
Indications :
- Hypoalbuminemia due to loss of albumin (burn injuries, nephrotic syndrome, etc.) and reduced albumin synthesis (liver cirrhosis, etc.).
- Hemorrhagic shock

Storage :
Do not freeze. Store at room temperature.
Shelf life :
3 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Ethanol fractionation
- Viral filtration
- Liquid heat treatment (60°C, 10h)
- Final product PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
Kenketsu Venilon-I I.V. Injection 500 mg, 1000 mg, 2500 mg, 5000 mg
(Freeze-dried Sulfonated Human Normal Immunoglobulin)
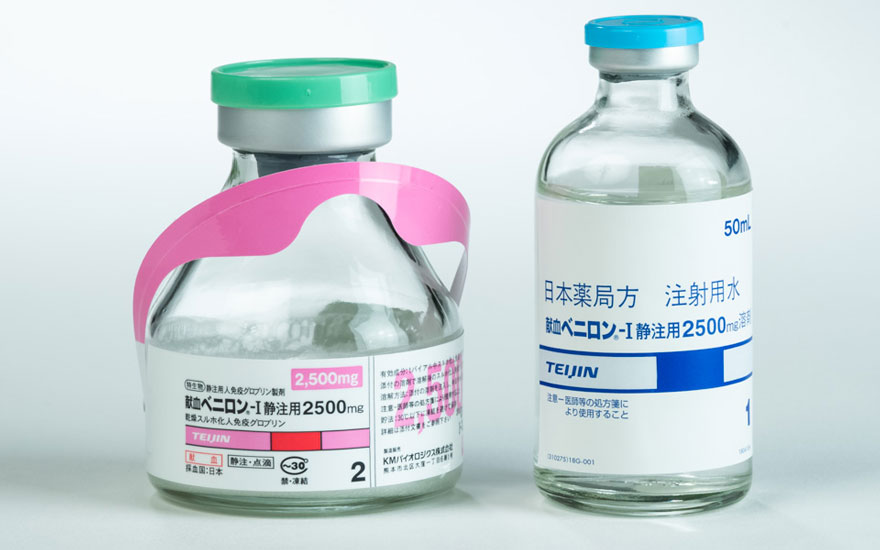
Indications :
- Hypogammaglobulinemia or agammaglobulinemia
- Combined use with antibiotics in severe infections
- Idiopathic thrombocytopenic purpura (when other medications are ineffective and there is a remarkable bleeding tendency and temporary hemostasis management such as surgical treatment or delivery is required)
- Acute Kawasaki disease (in severe cases with a risk of coronary artery dysfunction)
- Guillain-Barre syndrome (in severe cases causing difficulties walking in the acute exacerbation phase)
- Improvement of neuropathy in eosinophilic polyangiitis granulomatosis (only when steroid drugs are ineffective)
- Improvement of muscle weakness in chronic inflammatory demyelinating polyradiculoneuropathy (including multifocal motor neuropathy)
- Acute phase of optic neuritis (when steroids are ineffective)
Storage :
Store at 30°C or below.
Shelf life :
2 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Ethanol fractionation
- Sulfonation treatment
- Nanofiltratin
- Final product PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
Novact M I.V. Injection 500 units, 1000 units, 2000 units
(Freeze-dried Human Blood Coagulation Factor IX Concentrate)
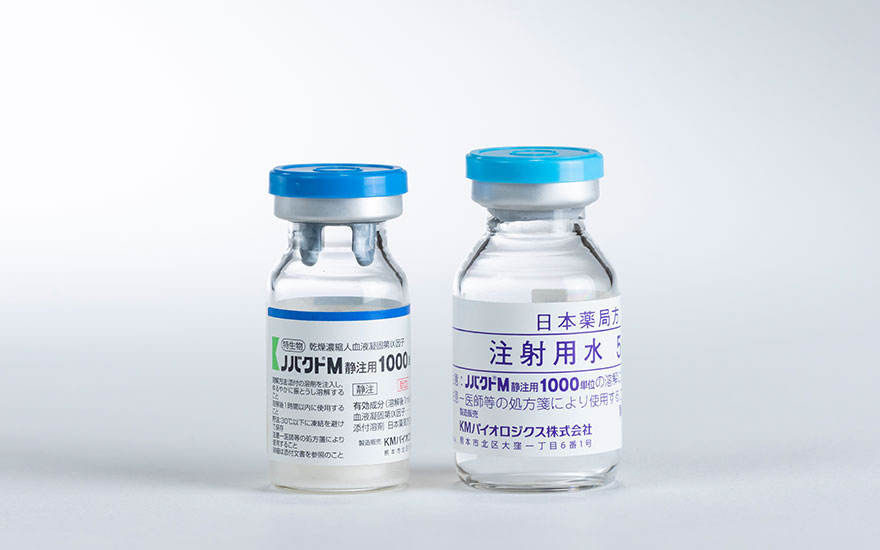
Indications :
Treatment and control of bleeding in patients with blood coaguration factor IX deficiency.
Storage :
Do not freeze. Store at 30°C or below.
Shelf life :
2 years
Virus inactivation/reduction :
- Blood from blood donors used as a row material of the plasma material is nagative for HBs-antigen, anti-HCV antibody, anti-HIV-1 antibody, anti-HIV-2 antibody, anti-HTLV-1 antibody and is screened for ALT value.
- Plasma material PCR (NAT) testing for HIV, HBV, HCV, and parvovirus B19.
- Immunoaffinity chromatography
- Viral filtration
- Dry heat treatment (65°C, 96h)
- Final product PCR (NAT) testing for HIV, HBV, HCV, HAV,and parvovirus B19.